‘What is molecular hydrogen?’
- 1.Biological characteristics of molecular hydrogen
- 2.Current state and prospect of medical research using molecular hydrogen
Biological characteristics of molecular hydrogen: biological activity of molecular hydrogen and its mechanisms, pharmacokinetics, intracellular kinetics, and safety thereof will be explained in this section.
1.1 What are reactive oxygen and free radical?
Oxygen occupies approximately 20 % of the air and is necessary in generating energy in breathing organisms. Once oxygen is taken into a body, it is taken into organelle called mitochondria and used to generate energy. However, on the other hand, several
percent of consumed oxygen becomes highly reactive, active oxygen. Within a human body, there are many types of reactive oxygen such as superoxide (O2-), hydrogen peroxide (H2O2),
hydroxyl radical (·OH) etc.
Everything including our bodies consists of molecules, and each of these molecules is constituted by a nucleus and electrons. An atomic element normally has a pair of two electrons existing in each electron orbit circuiting around a nucleus; however, there are cases where an electron is not paired (unpaired electron). Reactive oxygen, particularly kinds having this unpaired electron are collectively called free radicals. Since the unpaired electron has a property of wanting to become a pair, the free radical is unstable and greatly reactive.
1.2 Role of reactive oxygen
Reactive oxygen has both a beneficial side and a harmful side (that is, a ‘two-edged sword’) for living organisms. ·O2- and H2O2 exhibit a cytotoxic property at high concentrations,
but function as molecules for signaling at low concentrations, and play important roles for living organisms, such as apoptosis, cell proliferation, cell differentiation, etc. Furthermore, a high concentration of H2O2 is converted to hypochlorous acid (HOCl) by myeloperoxidase, and protects living organisms from bacterial attack. Moreover, nitric oxide (·NO), which is a type of reactive oxygen, is an important substance for intracellular signaling
and blood vessel dilation (Ohta S et al, 2012).
Meanwhile, of reactive oxygen, ?OH and peroxynitrite (ONOO-), which is produced from ·NO, has a harmful side. Particularly, as shown in FIG. 1-1, ·OH has 100 times more oxidizing power than ·O2- (Setsukinai K et al, 2003). These harmful types of reactive oxygen react with nucleic acid (DNA), lipids, and proteins that constitute our bodies, thereby oxidizing them. Oxidative stress induced by these types of reactive oxygen is a cause of cancer, various lifestyle-related diseases, aging, and the like.
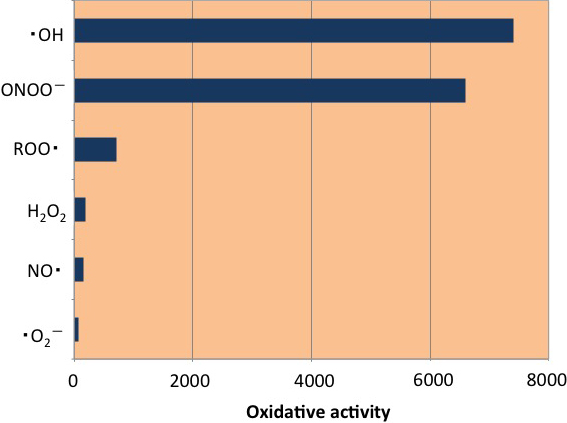
FIG. 1-1 Comparison of oxidative activities of reactive oxygen
(Modified citation from reference Setsukinai K, et al, 2003)
1.3 Biological activity of molecular hydrogen
MiZ considers the beneficial effects of ionized alkaline water made by an ionized alkaline water purifier (electrolyzed water purifier) on a living organism to be caused by molecular hydrogen (H2) produced in minute quantities
in the ionized alkaline water, and since around 1995, we have continuously carried out research and development of equipment and devices for effectively producing H2 and research of the antioxidant effect of H2 in a living organism, and have been patenting our technologies. That is, a pioneering research result that oral ingestion of neutral H2-dissolved water (1.6 ppm) manufactured in MiZ’s electrolytic cell in 2005 suppresses
oxidative stress disorder of a rat's liver that is induced by an oxidizing reagent called AAPH was presented in a thesis (Yanagihara T, et al, 2005). Moreover, it has been published in 2011 that oral ingestion of similar neutral H2-dissolved
water (1.4 ppm) by rats improved lipid oxidation markers, and that at the same time, expression of genes that contribute to oxidation-reduction in the liver was regulated, which was found through DNA microarray analysis (Nakai Y, et
al, 2011).
On the other hand, as an opposing research, a thesis by Ohsawa et al showing that inhalation of H2 gas by rats suppressed the ischemia-reperfusion injury of the brain as a model of cerebral infarction was released in 2007 (Ohsawa I, et al, 2007). The issue of this thesis was a turning point and it became widely known that H2 does not react with ·O2- and H2O2, which are important types of reactive oxygen to cells, but selectively reacts with ·OH and ONOO- that have particularly strong oxidizing power and reactivity and then neutralize them. It has become known that while substances with strong antioxidation (reducing power) such as vitamin C create strong oxidizing substances after the reductive reaction and damage DNA, H2 selectively removes only harmful reactive oxygen and converts it to water, and is thus very safe (Ohta S, et al, 2012).
1.4 Pharmacokinetics of molecular hydrogen
Molecular hydrogen (H2) that is dissolvable in water at normal temperature and pressure is approximately 0.8 mM (1.6 ppm). H2 is regularly produced in a living organism by intestinal bacteria of the large intestine.
The H2 produced by intestinal bacteria is emitted from the body as exhalation, or discharged from the anus along with other gases. When H2-dissolved water has been ingested, the H2 is mainly diffused
to tissue or organs adjacent to the stomach. However, it is inferred that a part of the H2 is absorbed from the gastrointestinal tract, circulated through the entire body via blood, and finally eliminated as exhalation.
MiZ has extensively studied the H2 concentration in tissue when H2 water (1.25~5 ppm) or H2 gas (1~4 %) is administered to rats by various administration routes (oral administration, intraperitoneal
administration, intravenous administration, and gas inhalation) (Liu C, et al, 2014). This study has shown that the concentrations of H2 in most of the tissue depend on the concentrations of administered H2. However,
at the same time, as shown in FIG. 1-2, since tissue having high concentration distribution differs according to administration route, the necessity of selecting the most appropriate administration route for the target organ or illness
has also been understood.
http://www.nature.com/articles/srep09629
http://www.nature.com/articles/srep05485#change-history
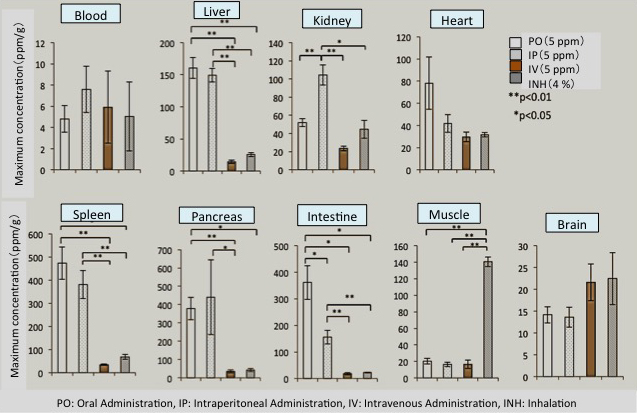
FIG. 1-2 Hydrogen concentrations in tissue in the case of administering molecular hydrogen via various administration routes
(Modified citation from reference Liu C, et al, 2014)
1.5 Intracellular kinetics of molecular hydrogen
The rate of diffusion of H2 in a living organism is by far faster than that of other substances. In other words, as shown in FIG. 1-3, H2 easily passes through a cell membrane, reaches the organelle, and reaches the nuclei responsible for intracellular genetic information and mitochondria that produces reactive oxygen, thereby exhibiting an antioxidant effect. On the other hand, it is reported that while vitamin A, vitamin C, and vitamin E are recognized as having an antioxidant effect, reachability of these substances to the organelle is poor since water-soluble vitamins (vitamin B, vitamin C, etc.) cannot pass through hydrophobic cell membrane, and fat-soluble vitamins (vitamin A, vitamin D, vitamin E, etc.) remain in the cell membrane (Ohta S, et al, 2012).
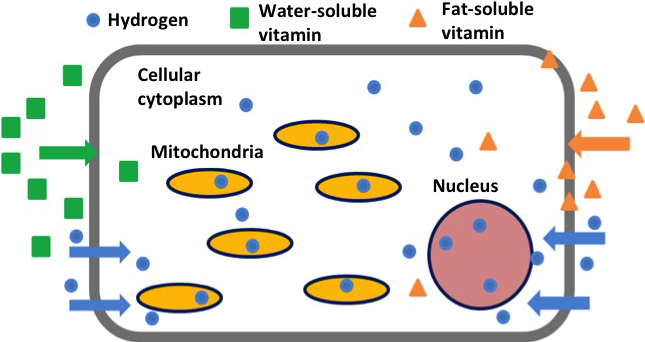
FIG. 1-3 Intracellular kinetics of molecular hydrogen and vitamins
(Modified citation from reference Ohta S, 2012)
1.6 Action mechanism of molecular hydrogen
H2 is reported having a variety of effects such as not only an antioxidant effect resulting from elimination of the active oxygen ·OH and ONOO-, but also an anti-inflammatory effect, an anti-apoptosis effect, an anti-allergic action, a lipid metabolism-improving effect, a neuroprotective effect, and an intracellular signaling regulatory effect, and there are currently approximately 740 original theses reporting the effects of H2. Just the removal of ·OH and ONOO- described in the previous section may not fully explain all of these effects. A part of the mechanism of H2 controlling intracellular signaling and gene expression has been revealed in recent research, and will be explained in detail in the following section.
1.7 Safety of molecular hydrogen
It is reported that H2 gas has a lower explosion limit of 4.7 % when reacting with oxygen, and a lower explosion limit of 4.0 % when reacting with air. However, there is no danger using a concentration of 10 % or less under the normal circumstances. No toxicity was found in a subchronic toxicity test where forced oral dosage (20 mL/kg/day) of H2 water was given to rats for 28 consecutive days (Saitoh Y, et al, 2010). Moreover, even clinical studies using humans did not show any adverse events (side effects) due to H2. H2 is a substance regularly produced in a living organism by intestinal bacteria of the large intestine, and thus does not harm our bodies. Furthermore, H2 gas has been used and mixed with oxygen gas and helium gas for prevention of caisson disease of divers in the deep sea through the ages. As a result, H2 is considered to be extremely safe.
1.8 References
- Kurokawa R, et al (2015); Med Gas Res, 5: 13.
- Liu C, et al (2014); Sci Rep, 4: 5485.
- Nakai, Y, et al (2011); Biosci Biotechnol Biochem, 75: 774-776.
- Ohsawa I, et al (2007); Nat Med, 13: 688-694.
- Ohta S. (2012); Biochim Biophys Acta, 1820: 586-594.
- Saitoh Y, et al (2010); Toxicol Ind Health, 26: 203-216.
- Setsukinai K et al (2003); J Biol Chem, 278: 3170-3175.
- Yanagihara T, et al (2005); Biosci Biotechnol Biochem, 69: 1985-1987.
